New Antibody Targets Tau Tangles
The next generation of drugs in the battle against Alzheimer’s disease may be tau protein-targeting antibodies. These proteins—which normally contribute to the health of neurons in the brain—are changed by Alzheimer’s and believed to then accelerate the disease. A new phase 1 study is seeking candidates to test one of the first of these new tau protein-targeting monoclonal antibody treatments.
Monoclonal antibodies mimic the body’s immune system by recognizing and binding to specific targets. To date, the treatments developed and approved for Alzheimer’s have focused on monoclonal antibodies that target amyloid proteins associated with the buildup of amyloid plaque in the brain. This build-up is thought to be one of the possible causes of Alzheimer’s. Laboratory research by Voyager Pharmaceuticals is instead focused on tau proteins.
“Tau cannot exist without the presence of amyloid,” said Dr. Jeffrey Norton, Medical Director at Charter Research’s offices in The Villages. “Amyloid would be much like the foundation of a house, and then the tau kind of builds upon that. So, the drugs work the same way, but they have different targets.”
A Different Approach
The buildup and clumping of amyloid plaque is a hallmark of Alzheimer’s disease, and current treatments focus on reducing this buildup in the brain. However, no treatments have stopped the disease.
“Current theories have been, ‘Okay, if removing amyloid from the brain slows the disease but doesn’t stop the disease completely, then tau must be part of the problem,’” Norton said. “That’s been a theory for some time. It is known that as tau burden—or amount—increases, that correlates to people deteriorating from Alzheimer’s. The next generation of drugs like this one from Voyager are saying, ‘Well, let’s see if we can work on that and slow the disease even better.’”
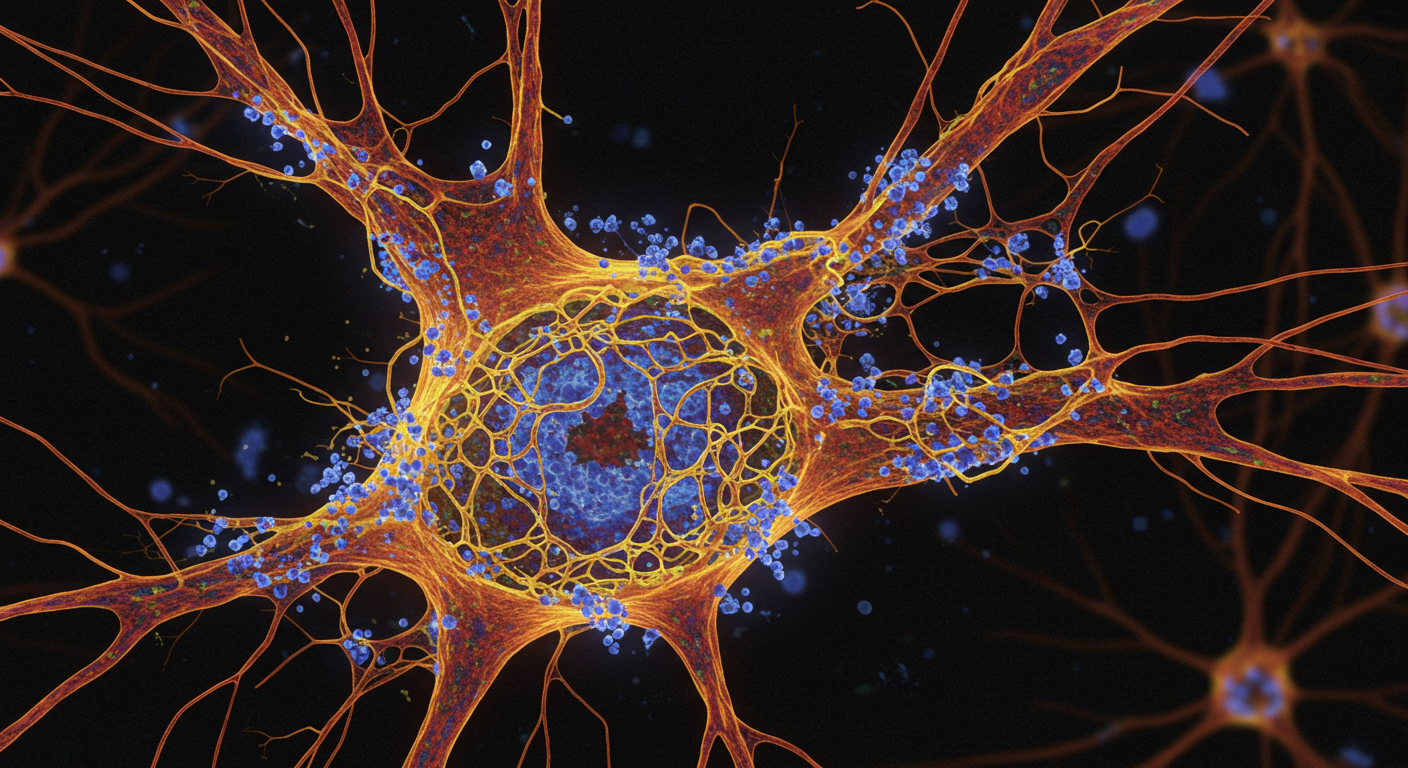
Voyager researchers believe the new drug, VY7523, is different from other tau protein-targeting antibodies because it targets the microtubule binding region (MTBR) of the tau protein. In healthy neurons, tau stabilizes the microtubules, which help to transport nutrients.
In Alzheimer’s disease, however, chemical changes cause tau to detach from the microtubules and clump together to form “tangles”. This then damages neurons and disrupts communication within—and between—them. This damage in key brain regions disrupts the brain’s circuits and networks responsible for various cognitive functions, possibly accelerating Alzheimer’s.
Laboratory tests by Voyager identified a specific antibody delivered intravenously inhibited the spread of tau tangles by more than 70%.
Study Structure & Intent
The trial aims to measure the safety and tolerability of VY7523. It will be split into three cohorts—groups of patients—each participating between 6–17 months. The placebo-controlled studies will test a range of escalating doses of the intravenous infusions on participants ranging from 50 to 90 years old.
All participants will undergo screening and baseline assessments. Cohorts 1 and 2 will then undergo five months of treatment, while cohort 3 will be in treatment for 17 months. All cohorts will conclude with end-of-study evaluations.
Norton said the time commitment for the Voyager trial for cohorts 1 and 2 will be less than one year in total, so it may be a good introduction for people considering participating in research. What’s more, the monthly infusions will take only about an hour.
“Phase 1B studies used to require people to stay overnight in a facility,” he said. “But drug companies have gotten much better at the way in which we monitor patient safety. They’ve been able to make it so that patients can do all of this on an outpatient basis.”
Help Shape Future Treatments
Participating in trials can be very rewarding and gratifying for participants who can say they played a role in getting a beneficial drug approved. This can be particularly true for people who have had other family members affected by the disease, Norton said.
“They don’t want to see anybody else go through what their family member went through,” he said.
In addition to the time commitment, the trial is seeking Alzheimer’s patients with early symptomatic disease defined both clinically (mild cognitive impairment) and by biomarkers (low to medium tau burden).
Participants also must have an identified reliable informant/caregiver, defined as someone who can support the participant for the duration of the study; accompany the participant to clinic visits; provide information to study investigators about functioning, cognitive abilities, and any adverse events; and support participants returning for follow-up visits and procedures.
If you’re interested in learning more, fill out this form or give us a call at one of our research centers:
Orlando 407-337-3000
The Villages 352-775-1000